| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
π Molecular Orbitals of Ethene
| Here the different phases of the lobes in the π orbitals are represented by the two different colours.
Notice that neither of the molecular orbitals are symmetric with respect to the internuclear axis and that the out of phase combination has an extra vertical nodal plane between the nuclei. |
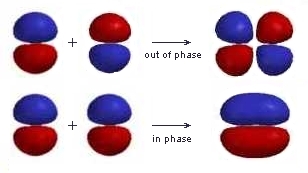 |
QUESTION: What type of molecular orbitals are symmetric with respect to the internuclear axis ? ANSWER
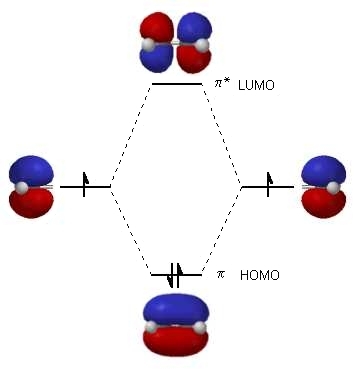
| The diagram to the right shows the relative energies
of the atomic p orbitals, the resulting π
molecular orbitals and the electron configuration.
(The same rules for filling orbitals applies to molecular orbitals as is used for atomic orbitals: review?) The two electrons from the atomic p orbitals are now paired in the stabilised π bonding orbital. This is the highest occupied molecular orbital or HOMO in ethene (or any simple alkene). In contrast, the π* anti-bonding orbital contains no electrons. It is the lowest unoccupied orbital or LUMO in ethene (or any simple alkene).
|
 |
| © Dr. Ian Hunt, Department of Chemistry |