| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
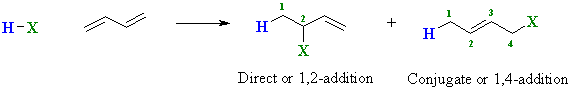
Addition of Hydrogen Halides to Dienes
Conjugated dienes undergo addition reactions in a similar
manner to simple alkenes, but two modes of addition are possible.
These differ based on the relative positions of H and X in the
products:
The distribution of the products depends on the reaction conditions as shown by the example below:
Direct H-X adds "directly" across the ends of a C=C Conjugate H-X adds across the ends of the conjugated system The numbers 1,2- and 1,4- denote the relative positions of H and X in the products
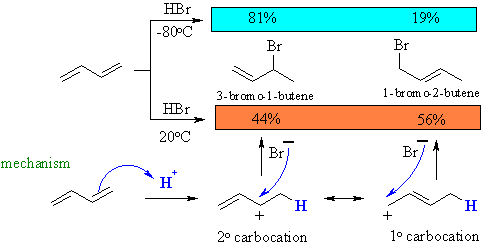
At low temperature, the reaction is under kinetic control (rate, irreversible conditions) and the major product is that from fastest reaction, that of the bromide with the secondary cation.
At higher temperature, the reaction is under thermodynamic control (equilibrium, reversible conditions) and the major product is the more stable system (note the more highly substituted alkene).
This is supported by the fact that heating pure samples of either 3-bromo-1-butene (direct addition product) or 1-bromo-2-butene (conjugate addition product) gives the same ratio of 3-bromo-1-butene to 1-bromo-2-butene.
| © Dr. Ian Hunt, Department of Chemistry |