|
|
2-butyne would react the first equivalent to give
2-bromo-2-butene. This would then react with a second equivalent of HBr
to give 2,2-dibromobutane. Both steps occur in accordance with Markovnikov's
rule (but based on carbocation stability). The protonation of 2-bromo-2-butene
preferentially gives the carbocation with the +ve charge on the C bearing
the Br - the Br is able to stablise the cation via resonance. 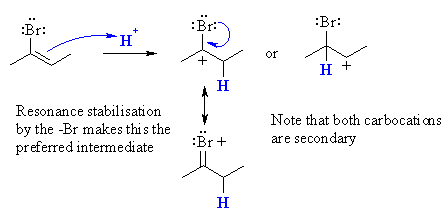 |