 |
Chapter 9 : Alkynes |
 |
Alkynes
Nomenclature:
Functional group suffix = -yne (review)
Disubstituted alkynes, R-C≡C-R', are described as "internal"
alkynes because the C≡C unit is "inside" the structure.
Monosubstituted alkynes, R-C≡C-H, and the unsubstituted alkyne (ethyne) H-C≡C-H are described as "terminal"
alkynes because the C≡C unit at the end of the structure.
|
|
|
|
|
|
|
ethyne (or acetylene)
|
|
propyne
|
|
terminal
|
|
terminal
|
|
|
|
|
|
|
|
1-butyne
|
|
2-butyne
|
|
terminal
|
|
internal
|
| Classify each of the following as an
internal or a terminal alkyne: |
| (a) 1-hexyne ANSWER |
(b) 3-octyne ANSWER |
| (c) cyclooctyne ANSWER |
(d) propyne ANSWER |
Stability:
- As with alkenes, the more highly substituted
internal alkynes are more stable.
- By comparing thermodynamic data of alkynes and alkenes,
it can be seen that the "extra" π bond in an alkyne is weaker than the
alkene π bond:
DHh 1-hexyne = 290 kJ/mol (69.2 kcal/mol) vs 1-hexene = 126
kJ/mol (30.2 kcal/mol)
So C≡C to C=C = 164 kJ/mol (39 kcal/mol) while C=C to C-C
= 126 kJ/mol (30.2 kcal/mol)
Therefore the "extra" π bond is 38 kJ/mol (8.8 kcal/mol) weaker that an alkene π
bond.
| Rank 1-hexyne and 3-hexyne for each of
the following properties: |
| (a) heat of hydrogenation |
ANSWER |
(c) heat of formation |
ANSWER |
| (b) heat of combustion |
ANSWER |
(d) stability |
ANSWER |
Structure:
|
|
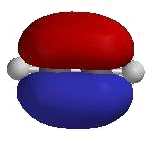
- The alkyne functional group consists of two sp
hybridised C atoms bonded to each other via one σ and two π
bonds.
- The 2 π bonds are produced by the side-to-side
overlap of the two pairs of p-orbitals on the C atoms not utilised in the hybrid set.
- The substituents are attached to the C≡C
via σ bonds.
- The 2 C of the C≡C and the 2 atoms
attached directly to the C≡C are linear.
- Since alkynes are linear, they cannot
exist as cis- / trans- isomers.
|
 |
 |
|
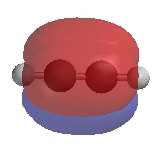 |
| The two separate perpendicular π
molecular orbitals |
|
Combined π molecular orbitals |
Physical Properties:
As with hydrocarbons in general, alkynes are non-polar and
are insoluble in water but soluble in non-polar organic solvents.
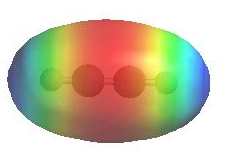
Reactivity:
- The π bonds are a region of high electron
density (red) so alkynes are typically
nucleophiles.
- Alkynes typically undergo
addition reactions in which one or both of the π-bonds are converted
to new σ bonds.
- Terminal alkynes, R-C≡C-H, are quite
acidic (indicated by blue) for
hydrocarbons, pKa = 26
- Deprotonation of a terminal acetylene gives an
acetylide ion.
- The acetylide ion is a good nucleophile and can
be alkylated to give higher alkynes.
|
 |



