|
Structure and Preparation of Alkenes. Elimination Reactions |
|
Structure and Preparation of Alkenes. Elimination Reactions |
E2 mechanism
E2 indicates an elimination, bimolecular
reaction, where rate = k [B][R-LG].
This implies that the rate determining step involves an interaction between
these two species, the base B, and the organic substrate, R-LG
This pathway is a concerted process with the following characteristics:
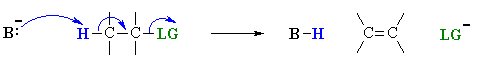
Let's look at how the various components of the reaction influence the reaction pathway:
Effects of R-
Reactivity order : (CH3)3C- >
(CH3)2CH- > CH3CH2-
> CH3-
In an E2 reaction, the reaction transforms 2 sp3 C atoms into sp2 C atoms. This moves the substituents further apart decreasing any steric interactions. So more highly substituted systems undergo E2 eliminations more rapidly. This is the same reactivity trend as seen in E1 reactions.
-LG
The C-LG bond is broken during the rate
determining step, so the rate does depend on the nature of the leaving group.
However, if a leaving group is too good, then an E1 reaction may result.
B
Since the base is involved in the rate determining step, the nature of the base
is very important in an E2 reaction.
More reactive bases will favour an E2 reaction.
Stereochemistry
E2 reactions occur most rapidly when the H-C
bond and C-LG bonds involved are
co-planar, most often at 180o or antiperiplanar. This sets up the
s bonds that are broken in the correct alignment to
become the p bond. More details ?
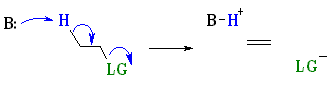
The E2 pathway is most common with:
| © Dr. Ian Hunt, Department of Chemistry |