| Chapter 4: Alcohols and Alkyl Halides |
| Chapter 4: Alcohols and Alkyl Halides |
Selectivity
There are two components to understanding the selectivity of radical halogenations of alkanes:
|
|
R-H |
|
|
Note how the bonds get weaker as we move down the table, so the R. also gets easier to form, with 3o being the easiest radical to form. |
|
|
CH3-H |
|
|
|
|
|
CH3CH2-H |
|
|
|
|
|
(CH3)2CH-H |
|
|
|
|
|
(CH3)3C-H |
|
|
Halogen radical, X.
The relative rates of reaction for X2 relative to chlorine are : F =108, Cl = 1, Br = 7 x 10-11 and I = 2 x 10-22 i.e. relative to chlorination, F reacts rapidly, Br very slowly and I very, very, very slowly.
For a given set of reaction conditions, the selectivity of the radical reactions can be predicted mathematically based on a combination of an experimentally determined reactivity factor, Ri, and a statistical factor, nHi.
Don't be intimidated by this equation, it's not as bad as you fear! In order to use the equation below, we need to look at our original alkane and look at each H in turn to see what product it would give if it were substituted. This is an exercise in recognising different types of hydrogen, something that will be important later.
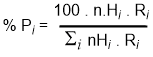 |
%Pi = % yield of product "i"
nHi = number of H of type "i" Ri = reactivity factor for type "i" Si = sum for all types |
Reactivity factors, Ri
|
What do the reactivity factors indicate ? Well as an example of the conclusions we could make:
Let's look at a worked example, say chlorination of propane, CH3CH2CH3
How many different monochlorides can be produced by radical chlorination ? ANSWER
This means there are two types of H atom in propane (use the JSMOL diagrams below to highlight this if you are unsure).
|
|
|||
|
|
|
|
Looking at the starting material alkane, i.e propane, we have two types of H:
(Don't make the mistake of looking at the number of types of H in the product that you are making, you need to look at the starting material)
Now for the calculations, so plugging the values into the equations we get (the reactivity factors Ri are in the table above):
% 1-chloropropane = 100 x (6 x 1) / (6 x 1 + 2 x 3.9) = 100 x 6 / 13.8 = 43.5 % (experimental = 44 %)What about bromination of propane ?% 2-chloropropane = 100 x (2 x 3.9) / (6 x 1 + 2 x 3.9) = 100 x 7.8 / 13.8 = 56.5 % (experimental = 56 %)
Most of the process in the same, all we have to do is change the reactivty factors
% 1-bromopropane = 100 x (6 x 1) / (6 x 1 + 2 x 82) = 100 x 6 / 170 = 3.5 % (experimental = 4 %)Note that the results match well with experimental values (under the same conditions) and that they illustrate the high regioselectivity of the bromination reaction for the 2o radical, whereas in the chlorination the number of 1o H dictates the regioslectivity.% 2-bromoopropane = 100 x (2 x 82) / (6 x 1 + 2 x 82) = 100 x 164 / 170 = 96.5 % (experimental = 96 %)
There are other examples in the sample problems.
| © Dr. Ian Hunt, Department of Chemistry |