| Chapter 2 : Alkanes |
| Chapter 2 : Alkanes |
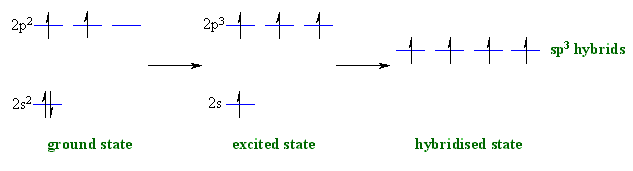
sp3 hybridisation
Now let's see how hydridisation
can account for each of these
features, working towards methane then other alkanes:
 |
|
|
|
|
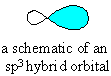
|
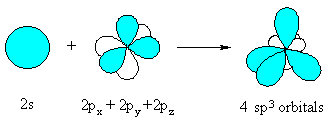
|
The sp3 hybrid orbital looks like a "distorted"
p orbital with unequal lobes.
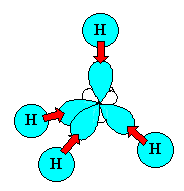
The 4 sp3 hybrids point towards the
corners of a tetrahedron. |
You can view an animation of the hybridisation of the C orbitals if you wish.
|
 |
|
 |
Summary
| © Dr. Ian Hunt, Department of Chemistry |