| Chapter 4: Resonance |
| Chapter 4: Resonance |
Ranking Resonance Structures
The following examples have been chosen to help illustrate some common mistakes and how to avoid them.
| 1. | 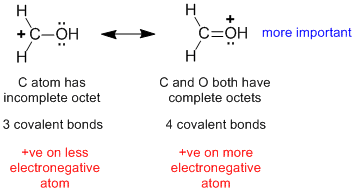 |
The mistake is to just look at the location of the +ve charge and the electronegativity of the associated atom (rule 4) and to overlook to octets at C and O (rule 1) and the number of covalent bonds (rule 2) which are more important factors. |
| 2. | 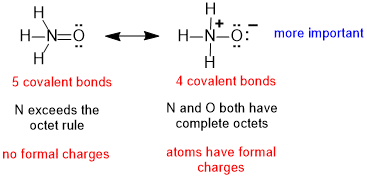 |
The mistake is to focus on the maximised number of covalent bonds (rule 2) and the absence of formal charges (rule 3) but overlook the fact that N can't form 5 bonds since N can not expand its octet and therefore the contributor on the left is not possible. |
| 3. | ||
| 4. | ||
| 5. | ||
| 6. |
 |
© Dr. Ian Hunt, Department of Chemistry |