| Chapter 3: Conformations of Alkanes and Cycloalkanes |
| Chapter 3: Conformations of Alkanes and Cycloalkanes |
Higher Alkanes
The same basic ideas of conformational analysis developed previously for simple alkanes also apply, but some new situations are encountered.
| In butane it is the rotation
about the C2-C3 bond that is of most interest since the relative position
of the two methyl groups is important.
This can be seen most easily by rotating the molecule to view it down the C2-C3 bond. Try rotating the 3D model in the animation so that you are looking directly along the C-C bond to see the interconversion of the two conformations. The more important conformations are shown below: |
|||
| ANTI a staggered conformation with the Me groups at 180o with respect to each other. This is the most stable conformation since the Me groups are as far apart as possible. |
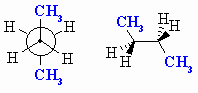 |
||
| GAUCHE a staggered conformation with the Me groups at 60o with respect to each other. |
 |
||
| SYN an eclipsed conformation with the Me groups at 0o with respect to each other. This is the least stable conformation due to the steric strain caused by the proximity of the Me groups and the torsional strain of the eclipsed bonds. |
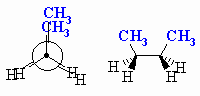 |
||
| GAUCHE a staggered conformation with the Me groups at 60o with respect to each other |
 |
||
|
|
|
||
The animation below shows how the potential energy of the
butane molecule varies for a full rotation about the central C-C bond.
Use the controls to show the energies of the important conformations.
|
QUESTION |
Hydrocarbons are
often drawn as simple "zig-zag" structures an example of which is given
below.
|
ANSWER |
 |
© Dr. Ian Hunt, Department of Chemistry |