| Useful Concepts |
| Useful Concepts |
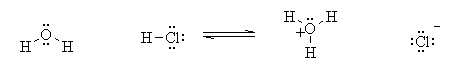 This is a simple acid / base reaction showing the formation of the hydronium
ion produced when hydrogen chloride is dissolved in water. In order
to develop an understanding of curly arrows, it is useful to analyse
the bond changes that are occuring.
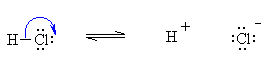 Next the reaction of the base with the proton requires that we make a new O-H bond. This requires that we put electrons BETWEEN the atoms that are to be BONDED:
So the curly arrows have accounted for all the bonding changes that occur in this process. We started with H2O and HCl we now have H3O+ and Cl- . However, we should consider this reaction as a single process where the BASE abstracts the PROTON from the ACID :
This is just the two separate steps rolled into one. Start from the lone pair on oxygen, make the O-H bond, need to break a bond (since H can only have 1 pair of electrons around it) so break the H-Cl bond giving the electrons to the electronegative Cl. QUESTION : Can you draw the curly arrows for the reverse process ? |