1.
|
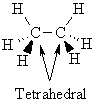
Give
a drawing that clearly indicates the shape
of ethane, CH3CH3, in 3D space.
On this diagram
indicate the electronic geometry
about all non-hydrogen atoms. |
|
|
|
|
2.
|
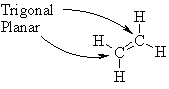
Give
a drawing that clearly indicates the shape
of ethene, CH2CH2, in 3D space.
On this diagram
indicate the electronic geometry
about all non-hydrogen atoms |
|
|
|
|
3.
|
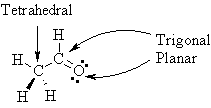
Give
a drawing that clearly indicates the shape
of ethanal, CH3CHO, in 3D space.
On this diagram
indicate the electronic geometry
about all non-hydrogen atoms. |
|
| Note how the
shape at the C that is part of the double bond is the
same here as it is in ethene above. |
 |
|
|
|
4.
|
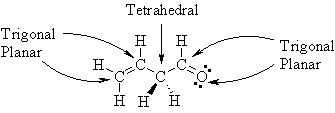
Give
a drawing that clearly indicates the orientation
of the atoms in 3-butenal, CH2=CHCH2CHO, in 3D
space.
On this diagram
indicate the electronic geometry
about all non-hydrogen atoms. |
|
| Note how the
shapes in this more complex system are a combination of
the pieces in questions 1, 2 and 3 above. |
 |
|
|
|
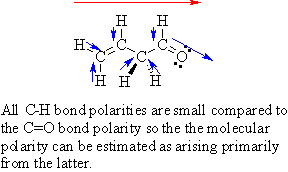
| 5. |
Using
a diagram, indicate the direction of molecular
polarity for 3-butenaldehyde. |
|
|


