Note that these types of questions are often the most difficult on the examination. This is because they are holistic in that they require a good understanding of the reactions and the concepts and the ability to apply those concepts from the course as a whole. They can't be memorised, they require understanding and ability to apply. Hence they help discriminate those who truly understand from those who have memorised and will soon forget.
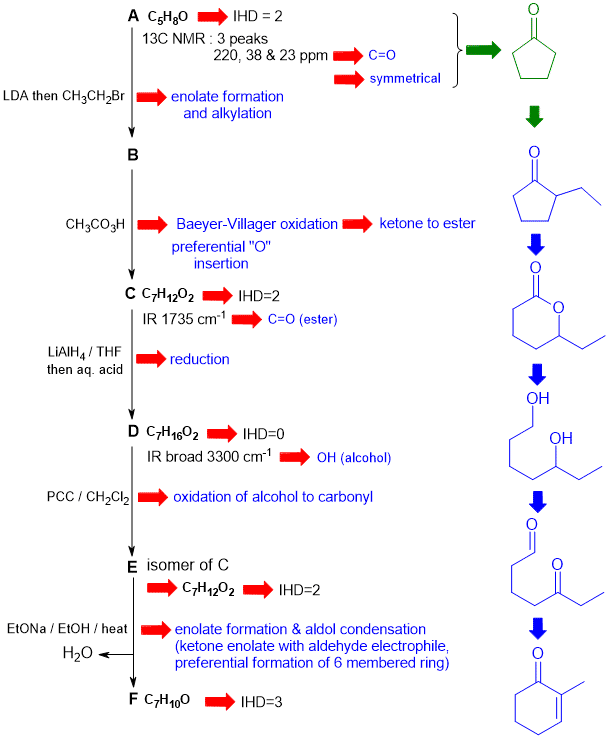
A schematic of the solution is shown below. The information from the question is given in black. Deductions directly from this information are indicated by red arrows. Points that provide potential key information are shown in green which leads to the structures...
In this problem key steps to success are:
1. A is symmetrical ketone (key structure) based on 13CNMR data.
2. Reactions are consistent with carbonyl chemistry (e.g. enolates formation and reaction, oxidation, reduction etc.
3. B --> C suggests Baeyer-Villager oxidation that goes on the more substituted side converting ketone to ester.
There are, of course, other possible thought pathways.
Common errors:
1. Not counting and/or numbering C atoms so making avoidable errors
2. Not knowing the reactions well enough (e.g. LDA is a base, commonly used to form enolates)
3. Not addressing the selectivity in the Baeyer-Villiger reaction (migratory preference H > 3oR > 2oR > Ar > 1oR > CH3 (less likely) which defines the cyclic ester C and hence diol D.