Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
Common errors : (1)
(2) not accounting for all bonding changes (3) balancing formal charges
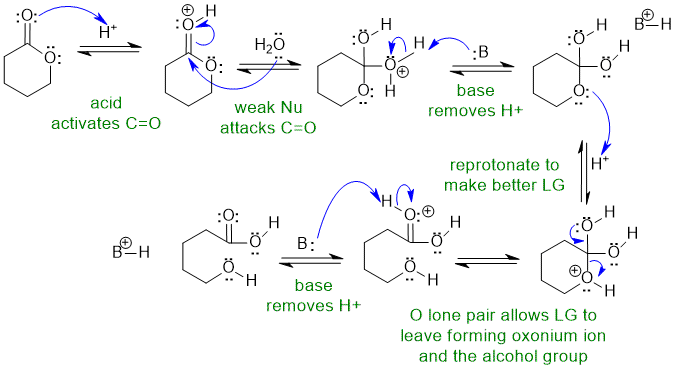
1A Acidic ester hydrolysis of a cyclic ester:

B: in this scheme could be a carbonyl group, ROH group, H2O or the conjugate base HSO4-
Common errors : (1) did not activate carbonyl to attack by weak Nu (2) used hydroxide as Nu despite acidic conditions (3) re-protonation arrows missing/incorrect (4) used "B" but did not identify it :(
1B Hydration of a nitrile:
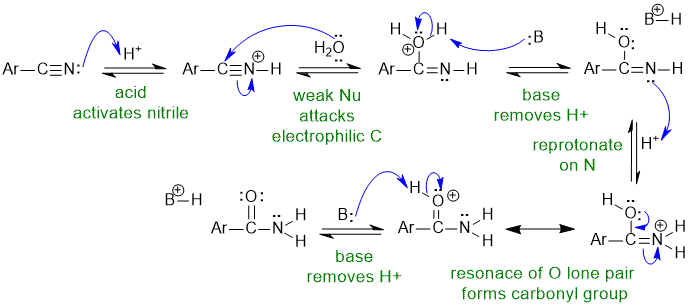
B: in this scheme could be the nitrile group, ROH group, H2O or the conjugate base HSO4-
Common errors : (1) did not activate nitrile to attack by weak Nu (2) used hydroxide as Nu despite acidic conditions (3) re-protonation arrows missing/incorrect (4) used "B" but did not identify it :(
2A A Dieckmann condensation:
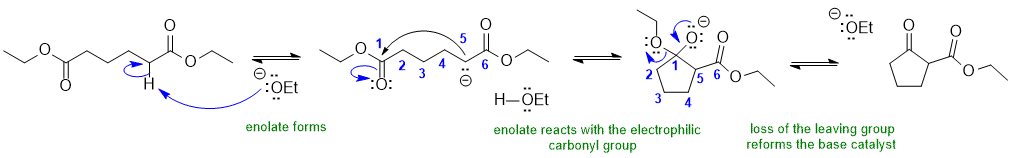
Common errors : (1) did not form enolate correctly (2) incorrect carbocycle ring size
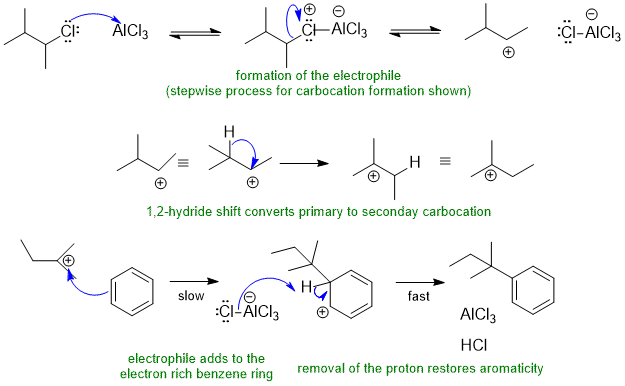
Common errors : (1) missed the hydride shift (carbocation rearrangement) (2) omitted the removal of the proton to rearomatise