Part 5: MECHANISMS
Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
General common errors:
(1) incorrect formal charges (2) backwards arrows (3) not showing the arrows for all the bonding changes (4) misuse of resonance / equilibrium arrows (5) vague arrows e.g. not starting where the electrons are i.e. on a bond or lone pair.
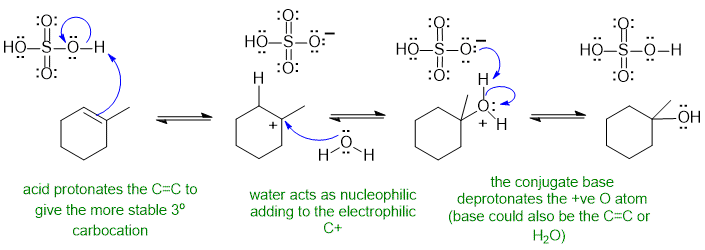
A1 Alkene hydration (follows Markovnikov's rule):

Common errors : Using HO- as the nucleophile despite the acidic reaction conditions, not reforming the acid catalyst
A2
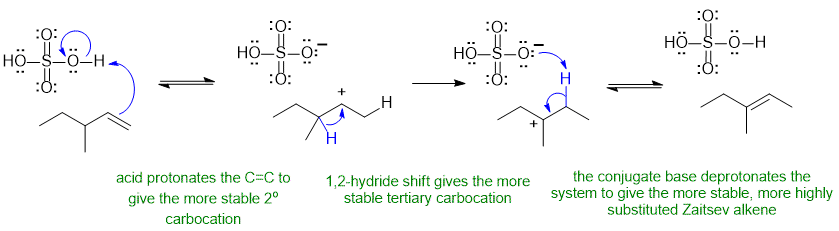
Common errors : not reforming the acid catalyst, missing the rearrangement
B1 An intramolecular Diels-Alder reaction :

The starting material contains a conjugated diene (C1-C4) with two electron donating alkyl groups and an alkene dienophile (C8-C9) with an ester electron withdrawing group attached.
Common errors : no justification (read question), lack of understanding of the Diels-Alder reaction which generates substituted cyclohexenes
B2 An aldol condensation (covered in two laboratory activities pre-MT in W22) one of which specifically reviewed all the steps of this mechanism:
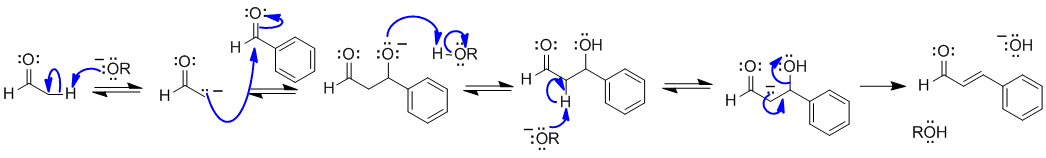
The reaction is an aldol condenstation where the ethanal must form the enolate (only species with alpha-H). The conjugated nature of the product favours the elimination (E1cb) to make the dehydrated product.
Common errors : did not show the formation of the enolate, didn't recognise the nucleophilic and the electrophilic species, "exotic intermediates" utilising reactions and steps that don't resemble 351/3 organic chemistry...