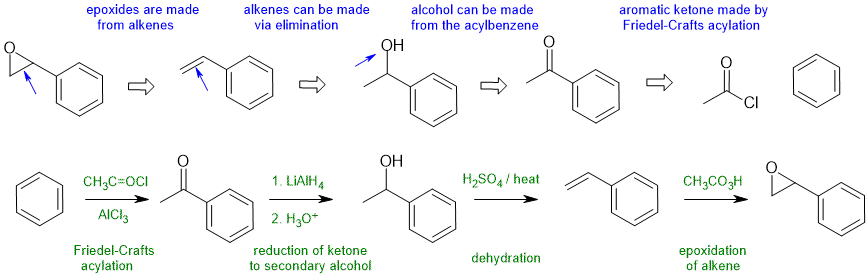
Answers to the synthesis problems are given below. These answers have been selected as they are short and efficient, but there are probably other reasonable solutions. Note some targets have specific stereochemistry that needs to be considered.
When planning syntheses, one should try to work backwards by functional group analysis. The diagrams show the "retrosynthesis" - the design or plan and then below that the reaction scheme step-by-step (as required in the question). Red arrows try to show carbon-carbon bond forming reactions, blue arrows are functional group manipulations. The blue text rationalise the retrosynthetic analysis and information about important reactions are given in green.
A1

A2
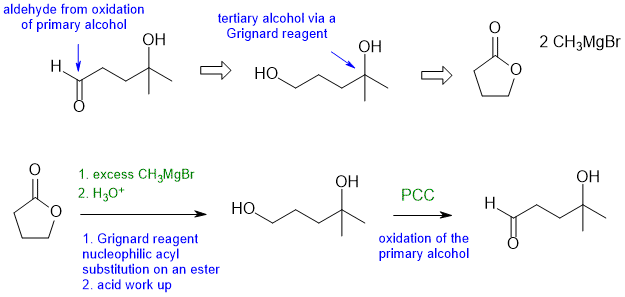
Common errors : relative reactivity of aldehyde > ketone > ester towards Grignards
B1

Common errors : missing steps in diazotisation sequence
B2

Common errors : not using a protecting group for the ketone, trying to do Friedel-Crafts on a deactivated system