Note that these types of questions are often the most difficult on the examination. This is because they are holistic in that they require a good understanding of the reactions and the concepts and the ability to apply those concepts from the course as a whole. They can't be memorised, they require understanding and ability to apply. Hence they help discriminate those who truly understand from those who have memorised and will soon forget.
A schematic of the solution is shown below. The information from the question is given in black. Deductions directly from this information are indicated by red arrows. Points that provide potential key information are shown in green which leads to the structures....
In this problem key steps to success are:
1. Drawing out methoxybenzene and recognising that it unders para substitution adding a alkyl group with 4C but only 2 types of C = must be a t-butyl group.
2. A is C4H8 which with an IHD of 1 means 6 possible structures but reaction data means A is an alkene (4 possible structures).
3. The "carbonyl compound" must be an aldehyde or a ketone (only 2 possible structures) : CH3CH2CHO or (CH3)2C=O giving two possible alkenes CH3CH2CH=CH2 or (CH3)2C=CH2 respectively. Only one of this alkenes can give rise to the t-butyl group via the other reactions in the scheme.
4. The H NMR for B can be used to deduce the structure of B.
There are, of course, other possible thought pathways.
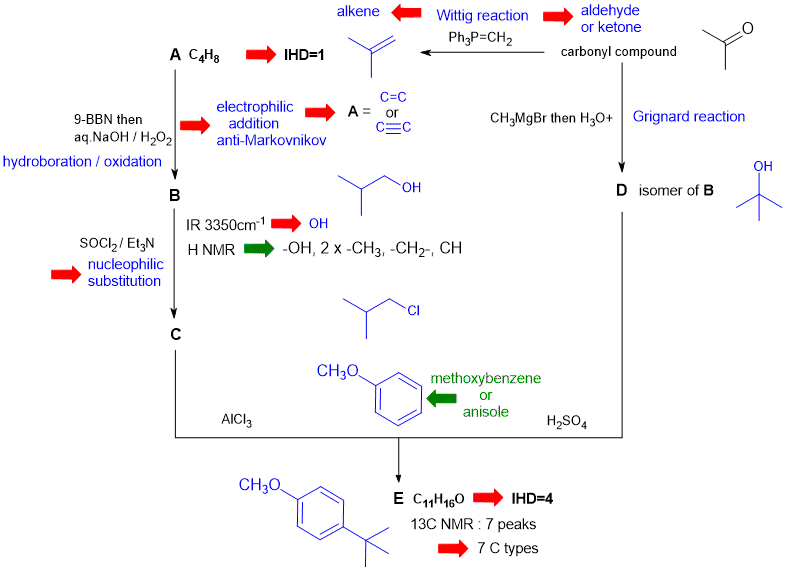
Both C and D lead to the same product because in the reaction of C with AlCl3 (Friedel-Crafts alkylation) the C+ intermediate will undergo a 1,2-hydride shift to generate the required tertiary C+. In the reaction of D with H2SO4, the tertiary alcohol loses H2O to form the same tertiary C+. (3%)
Common errors:
1. Not recognising that A needed to have a branched structure.
2. Incomplete explanation of how C AND D lead to E (you need to explain how each of C and D form the same electrophile).
3. Incorrect structure for methoxybenzene (simple nomenclature).
4. Not knowing the details of some of the reactions involved (e.g. hydroboration, SOCl2...)