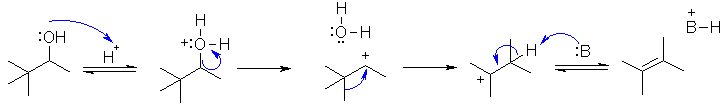
Part 6: MECHANISMS
Note that no other reagents are needed in order to complete any of these sequences, you
should only be using what is there.
A.
This is an acid
catalysed dehydration of an alcohol. with a C+ rearrangement. The first step is
protonation of the -OH with the H+ to give the leaving group. Loss of the leaving
group gives the 2o carbocation.
This rearranges via a 1,2-methyl shift to give the more stable 3o carbocatio.
Loss of the proton gives the product.
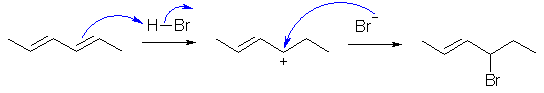
C.
The conjugated diene undergoes direct or 1,2-addition under kinetic
control in accord with Markovnikov's rule to give the product shown. Reaction
goes via protonation to give the more stable carbocation.
Mono substitution is the product because the diene is more reactive than the
product alkene since it is more electron rich (more nucleophilic).
![[Chem 350 Home]](mol.gif) Return
to Homepage
Return
to Homepage