
Part 5: STRUCTURE DETERMINATION
Note that there is no point showing all possible answers here, so the most likely and potentially simplest answers are shown.
a. C6H10O2 has a molecular weight (6 x 12.01) + (10 x 1.008) + (2 x 16.00) = 114.14 g/mol (1.5 marks)
b. The formula for index of hydrogen deficiency, IHD = 0.5 ( 2c+2-h), therefore, the IHD = 0.5 (2 x 6 + 2 - 10) = 2 (1.5 marks)
c. A pKa of 25 would suggest either a terminal alkyne or an ester is present :

(2 marks)
d. IR of 3300cm-1 suggests an -OH and soluble in NaOH solution suggests a carboxylic acid (this relates to the solubility experiment). 5 types of C requires some symmetry. (2 marks)
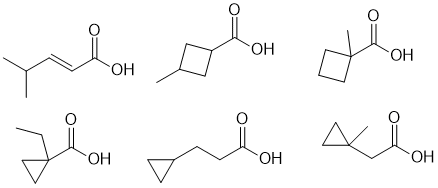
e. No IR of 3300-3150 cm-1 means no -OH and not a terminal alkyne, and a peak between 2300-2100 cm-1 means it is an alkyne so it must be internal: (2 marks)
![]()
f. Need an ester, alkene and chiral center (4 marks)
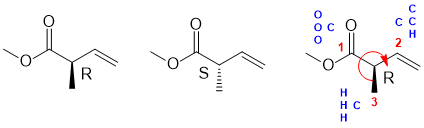
Common errors: