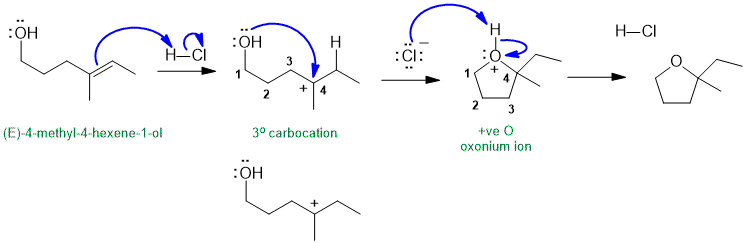
Part 6: MECHANISM
The following diagram shows the solution to the mechanistic question. Note that all the information applies to a single reaction sequence that has been completely described verbally. There is no need for extra reagents or extra steps etc. The curly arrows are drawn specifically to match the text in the question. The biggest problem students have is making sure they understand the language of chemistry. Most students have trouble because they can't draw the structures from the IUPAC names (that means they don't know their nomenclature well enough). Read the words carefully, and then make the curly arrows tell that same story. There is NO need for extra steps. Remember curly arrows go from electron rich to poor and to balance the formal charges at each step - errors on formal charges were common.
a.

(marks for correct structures, curly arrows, formal charges)
If you struggled with this part of the question, first draw the compounds whose names were provided, then think about the types of reactions (e.g. acid / base) and try to fill in the structures in the gaps, then finally add the required curly arrows to account for all the bonding changes.
In the image shown above note how the charges balance at each step, how the arrows start and end very close to the structures.

We also saw what I describe as "bouncing arrows". These are not consistent with the way organic chemists use arrows and they have the potential to become too confusing and they should not be used. They would imply that the e from the C=C pi bond move to one of the C atoms first and then on to make a new bond. That isn't what happens !
b. The image belows show a couple of the possible wedge hash representations. Note how the wedge and hash bonds are both shown since all the substituents are other than H and that the wedge and hash are shown adjacent to ensure the tetrahedral shape is unambiguous. The working for determining the configuration is also shown. Note that the reaction will produce BOTH on these enantiomeric structures.

(3 marks : 1 for wedge hash, 1 for priorities, 1 for correct R/S)
c. The Z isomer would give the same product because protonation of the C=C gives the same intermediate tertiary carbocation: (2.5 marks :1 for "same" product, 1.5 for why)
Note that we saw some structures that are problematic...
![]()
Compare this with the one shown above. There are two concerns here. First the representation of the -OH in the alcohol implies that the H is between the C and the O. In reality it is not, it is at the end on the O. The problem with this representation is that is going to cause confusion later and therefore it should be avoided. Second, this representation of the alkene unit is unacceptable as it is ambiguous in terms of stereochemistry. It is important to draw molecules so they look like they are in real terms (see the alkene in the structure above).
Common errors: