Part 7: MECHANISMS
Note that no other reagents are needed in order to complete any
of these sequences, you should only be using what is there.
I Radical halogenation reactions.
First step is the formation of the halogen radical by dissociation of the
halogen. Note that the Br-Br bond is weak and readily broken when heated.
T
he Br radical then abstracts the
tertiary benzylic H to give the resonance stablised tertiary radical and this
in turn leads to the tertiary bromide. Note that a bromine radical
is also produced and this means the process can continue (it's a cyclic process).
Note that because we are talking about radicals, we need to use
fishhook
type curly arrows.

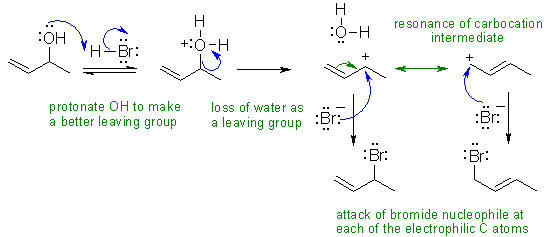
II The reaction of an alcohol
with a hydrogen halide such as HBr is typically SN1
in character provided a reasonable carbocation is being formed. Here, thats
means a resonance stabilised secondary carbocation. The two products arise due
to the possibility of the bromide nucleophile attacking either of the electrophilic
carbocations revealed by the resonance contributors.
III If an alkyl
bromide is heated with strong base (here it's t-butoxide), then a 1,2-elimination
to give an alkene occurs. Alkyl
halide eliminations are typically E2. In this case the base is quite large
so anti-Zaistev elimination is going to dominate.

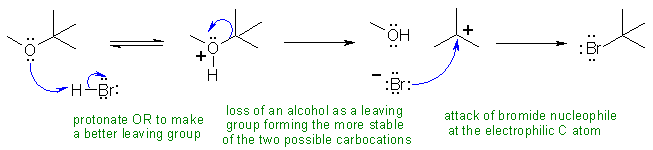
IV Ethers
can be cleaved by strong acids like HBr via an SN1 type reaction to give
an alcohol and an alkyl halide. The selectivity is controlled by the stability
of the intermediate carbocation.