
a.
Elemental
analysis :
The data doesn't add up to 100%, it the absence of other data we should assume
that oxygen makes up the rest (this is because oxygen can't be measured in a
combustion analysis). Note there is a hint is the last part of the question
when it mentions the aldehyde.
%C = 66.61 divide by atomic weight = 66.61 / 12.011 = 5.546
%H = 11.19 divide by atomic weight = 11.19 / 1.008 = 11.101
%O = 27.55 divide by atomic weight = 22.20 / 15.999 = 1.388
Now divide by the smallest to get the simplest ratio : C = 5.546 / 1.388 = 3.996,
H = 11.101 / 1.388 = 7.998, O = 1.388 / 1.388 = 1.000 Remember to be very careful
when rounding during during EA calculations (it will invariably mean you get
the wrong answer if you round too far) Therefore molecular formula = C4H8O.
b.
Index of hydrogen deficiency ? Either draw out
an example and count the pi bonds and rings, or use the formula....
IHD = 1
c.
The two possible correct answers are shown below along with the most common
incorrect answer:

d.
Enantiomers are non-superimposable mirror images, for that they need a chirality
center, typically an sp3 C with 4 different substituents attached.
The biggest error was drawing a molecule that lacked a chirality center or didn't
show it in 3D (they are stereoisomers after all). Then you need to assign
as R or
S. You only have to draw one pair, the most common answers are shown
here:
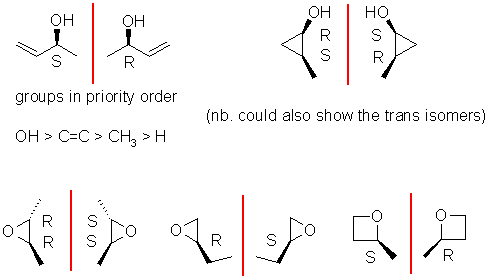
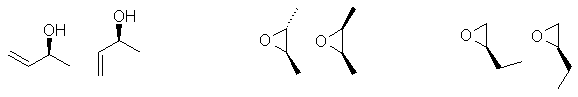
e. Diastereomers are stereoisomers that are not enantiomers. Lots of people drew constitutional isomers instead of diastereomers. Two possible answers.... if you had more than one chirality center then draw a different configuration (but not just the enantiomer - see the middle example) or draw a different conformer (the first and third examples). You only need to draw one diastereomer, various examples are shown here.
f. There are two possible aldehydes with this formula, but only one with three types of carbon :

Common stumbling blocks for some students were :