Part 6: SYNTHESIS
Answers to the synthesis problems are given below.
These answers have been selected as they are short but there are probably other
reasonable / alternative solutions ..... Of course they are based on the reactions
covered in the Fall semester !
Common errrors:
- Using the wrong reagents e.g. base to eliminate alcohols or acids
to eliminate halides, or HX to radically halogenate alkanes.
- Not showing how to prepare CH3CH2SH or CH3CH2Br (depending to the route
used) in B since the question asked and not starting the syntheses
from hydrocarbons as specifically
asked for in the question.
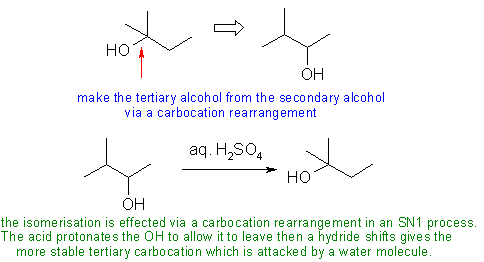
- In C using several steps where one would work, not forcing SN1 to
get the C+ rearrangement.
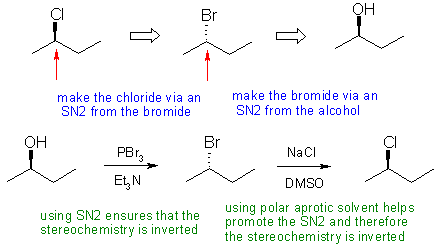
- In D, not paying attention to the stereochemistry of the product
or of the reaction steps. SN1 will lead to the racemized product and SN2 inversion,
so we need a double inversion, i.e. two reactions that each
cause inversion. Using alcohol to tosylate to chloride will give the opposite
stereochemistry to that required because the tosylate has the same stereochemistry
as the original alcohol.