The following data is available from the question:
MS: M = 164 g/mol
EA:
Calculates
empirical formula = C5H6O (IR confirms O is
present).
Which with MW from MS gives molecular formula = C10H12O2
. From here we get the IHD
= 5
IR: There are significant absorptions at 1710cm-1 due to a C=O and at 1600cm-1 due to aC=C, C-O possible due to bands at 1275 and 1100cm-1. There is no OH (about 3500cm-1).
13C nmr: The proton decoupled spectrum shows a total of 8 peaks indicating 8 types of C. Since we know the formula, we know that since there are 8C from the MF then there is some symmetry. By analysis of the chemical shifts, we have a C=O (probably an acid derivative) at 167 ppm, 4 types in the region 125-140 ppm is typical of aromatic C, 60 ppm is typical of a C-O and those at 22 and 14 ppm most likely from hydrocarbon.
1H
nmr: First of
all we have 5 types of H showing up. After this, it's a good idea to
tabluate
the information to make sure you get it all correctly matched up:
|
|
|
|
|
|
|
|
|
Ar-H, must be disubstituted, most likely para |
|
|
|
|
|
|
|
|
|
CH2 coupled to 3H, deshielded by O ? |
|
|
|
|
CH3 with no adjacent H, slightly deshielded |
|
|
|
|
CH3 coupled to 2H |
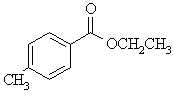
The most significant structural information in the H nmr data is that we have a disubstituted phenyl group, an ethyl group : -CH2-CH3 most likely as an ethoxy group: -O-CH2-CH3 and an isolated methyl group : -CH3
The IR gave us the C=O which the C nmr suggests is an acid derviative, such as an ester rather than a ketone (typically > 200ppm).
So with all this information we have the following pieces: C=O, -O-CH2-CH2- , -CH3 and C6H4-.
Altogther...

ethyl 4-methybenzoate. The -CH2-
is deshielded by O and coupled
to the -CH3, while the other -CH3 is isolated and
slightly deshielded by the phenyl ring. The number of types of ArC (4)
and the coupling in the Ar region of the H nmr (7-8ppm) suggests the
para
substitution pattern.
The final step should always be to check
what you have drawn. The easiest
thing to check is usually the coupling patterns you would expect to
see,
and the chemical shifts of each unit.
You should be asking yourself : "Does my answer give me what the
H-nmr shows ?"