| Useful Concepts |
| Useful Concepts |
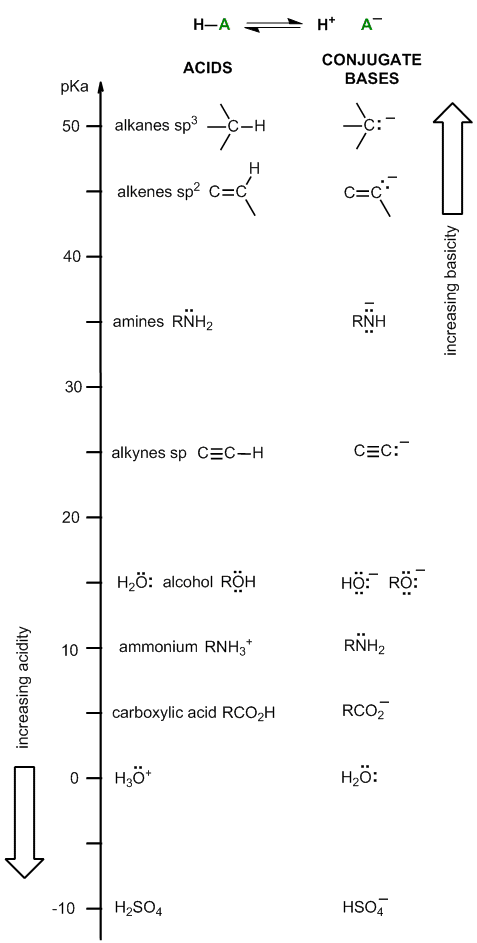
Acidity ladder (part 2)
This version of the ladder can help give a better understanding of "how it works" or how to use it correctly.
First remember the fundamental equation for acidity, HA <=> H+ + A-, in this version of the ladder both HA and A- (the conjugate base) are shown.
When thinking in this way, one gets a better appreciation for why compounds like water and amines appear "twice", once in the acids column and once in the conjugate base column. Grasping this can help make sure you get the right pKa!
This version of the ladder may also help you get the ideas around the fact that a strong acid (such as H2SO4) had a weak conjugate base (HSO4-) organised...
Using the figure...
(1) What bases will deprotonate a carboxylic acid >99% when used in equimolar amounts ?
Find the ACID, the carboxylic acid, RCO2H, in the H-A (acids) column (pKa =5). Move horizontally across to the right to the A- (conjugate bases) column.
Any conjugate base above that will give >99% deprotonation (e.g. amines RNH2, hydroxide HO- or alkoxides RO-, etc. )
(2) What acids will protonate an amine >99% when used in equimolar amounts ?
Find the BASE, the amine, RNH2, in the A- (conjugate bases) column (pKa =10). Move horizontally across to the left to the HA (acids) column.
Any conjugate acid below that will give >99% deprotonation (e.g. carboxylic acids, RCO2H, hydronium H3O+ or sulfuric acid H2SO4, etc. )
| © Dr. Ian Hunt, Department of Chemistry |