|
|
|
|
Nucleophilic Addition
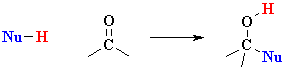
What does the term "nucleophilic addition" imply ?
A nucleophile, Nu-, is an electron rich species that will react with an electron poor species (here the C=O)There are three fundamental events in a nucleophilic addition reaction:
An addition implies that two systems combine to a single entity.

Examples of such nucleophilic systems are : RMgX, RLi, RC≡CM,
LiAlH4, NaBH4

| The protonation of a carbonyl gives a structure that can be redrawn in another resonance form that reveals the electrophilic character of the C since it is a carbocation. |  |
The reactions on the following pages
are catalogued based on the nature of the nucleophilic atom involved.
In most cases it is useful to indentify the mechanism in each case in terms
of the two general schemes presented above.
| © Dr. Ian Hunt, Department of Chemistry |