| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
Kinetic and Thermodynamic Control
The potential outcome of a reaction is usually influenced by two factors:
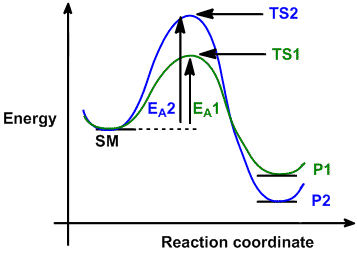
| Consider the case where a starting material, SM,
can react in a similar manner to give two different products, P1 and P2 via
different (competing) pathways represented by green and blue curves.
Reaction 1 via pathway 1 (green) generates product 1 (P1) via transition state 1 (TS1). Reaction 2 via pathway 2
(blue) generates product 2 (P2) via
transition state 2 (TS2). |
 |
We now need to consider how the outcome of this situation changes with the competing reactions of the starting material as we alter the reaction temperature and therefore the average energy of the molecules changes.
1. At low temperature, the average energy of the molecules is low and more molecules have enough sufficient energy cross activation energy EA1 than EA2. Therefore the reaction preferentially proceeds along the green path to P1. The reaction is not reversible since the molecules lack sufficient energy to reverse to SM, i.e. it is irreversible, so the product ratio of the reaction is dictated by the rates of formation of P1 and P2, k1: k2.
2. At some slightly higher temperature, reaction 1 will become reversible when sufficient molecules have enough energy to cross the reverse reaction barrier for reaction 1, while reaction 2 remains irreversible. So although P1 may form initially, over time it will revert to SM and react to give the more stable P2.
3. At high temperature, both reaction 1 and 2 are reversible and the product ratio of the reaction is dictated by the equilibrium constants for P1 and P2, K1 : K2.
Summary
| © Dr. Ian Hunt, Department of Chemistry |